Lithium iron phosphate batteries and ternary lithium batteries in lithium batteries have the advantages of high energy density, wide operating temperature range, long cycle life, and safety and reliability. They are widely used as power batteries for new energy vehicles. However, during the charging and discharging process of lithium batteries, reversible reaction heat, ohmic heat, polarization heat, and side reaction heat are generated. The heat production of the battery is mainly affected by its internal resistance and charging current.
Power batteries are very “delicate.” Temperature has a very significant impact on the overall performance of power batteries, mainly reflected in three aspects: performance, lifespan, and safety. In the application of electric vehicles, it is generally necessary to comprehensively consider the impact of temperature on battery performance, lifespan, and safety to determine the optimal operating range of the battery and to achieve the best balance between performance and lifespan within this temperature range. It is generally believed that the optimal operating temperature range for batteries is 20°C to 30°C. In actual projects, it is necessary to determine the optimal operating temperature of the battery based on the related thermal test results of the battery.
The capacity of lithium batteries changes with temperature. Through testing, it has been found that for every 1°C increase in temperature, the capacity increases by 0.8% of the original. However, the increase in temperature can also damage the battery, gradually reducing the battery’s cycle life and capacity. According to experiments, in a normal temperature environment of 25°C, if the temperature rises by 6 to 10°C, the battery’s lifespan will be halved due to the increased floating charge current caused by high temperatures. Due to the accumulation of overcharge, the battery’s cycle life is shortened.
The capacity of lithium batteries increases with the rise in temperature. If the battery temperature rises and the total discharge remains unchanged, the discharge depth will decrease. When the battery temperature rises to 45°C, it can extend the service life. If the battery is charged at a temperature above 50°C, the acid will accelerate the corrosion on the battery plates, and the increase in temperature will accelerate the aging of the battery housing.
Changes in temperature cause varying degrees of attenuation in the available capacity of lithium batteries. The specific reference levels are: -10°C with 70% available capacity, 0°C with 85% available capacity, and 25°C with 100% available capacity. Therefore, it is normal for battery performance to decline in cold weather. When the temperature drops, the battery discharge voltage also decreases significantly, causing the battery to reach the discharge cut-off voltage faster during low-temperature discharge, resulting in a significantly lower low-temperature discharge capacity compared to normal temperature capacity.
When lithium-ion batteries are at low temperatures, their available capacity decreases and the power for charging and discharging is limited. If power is not restricted, it can cause the precipitation of lithium ions inside the battery, leading to irreversible capacity decay and potential safety hazards. The lower the environmental temperature, the lower the activity of the active materials within the battery, the higher the internal resistance and viscosity of the electrolyte, and the more difficult the ion diffusion. At low temperatures, the diffusion speed of lithium ions in the electrodes is slow, making it harder for them to intercalate and easier to deintercalate, causing a rapid decline in capacity. Therefore, using batteries at low temperatures can greatly affect their lifespan.
It is likely that everyone has experienced that lithium batteries last longer in the summer than in the winter. This demonstrates that the performance of lithium batteries is affected by the ambient temperature. Among all environmental factors, temperature has the greatest impact on the charging and discharging performance of lithium batteries. It is commonly known in the lithium battery industry that temperature changes significantly affect the stability of the charge and discharge state of lithium batteries. When lithium batteries are charged and discharged at high or low temperatures, the capacity retention rate decreases.
It is important to clarify that the capacity of lithium-ion batteries at low temperatures does not disappear but is simply unable to be fully released within the normal voltage range (≥3.0V). If the discharge cut-off voltage can be further lowered, the remaining capacity can be discharged.
The electrochemical reactions at the electrode/electrolyte interface are related to the ambient temperature, and this interface is considered the heart of the battery. If the temperature drops, the reaction rate of the electrode also decreases. Assuming the battery voltage remains constant, the discharge current decreases, and the power output of the battery will also decline.
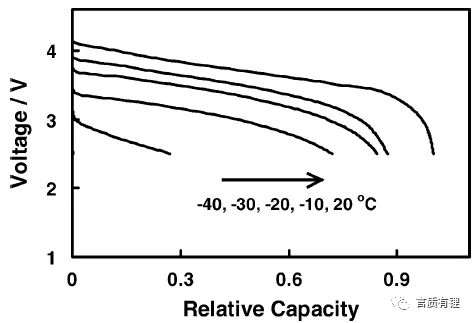
Figure 1 is a schematic diagram of the discharge capacity curve of lithium-ion batteries at different low temperatures (used here to indicate a general trend). Compared to room temperature at 20°C, the capacity loss is already quite noticeable at -20°C, and at -30°C, even more capacity is lost. At -40°C, the capacity is less than half.
Let’s take a look at the factors affecting low-temperature performance. By comparing the relationship between capacity and electrolyte conductivity (Figure 2), it can be seen that the lower the temperature, the lower the conductivity of the battery electrolyte. After the conductivity decreases, the ability of the solution to conduct active ions decreases, which manifests as an increase in the resistance of the internal reactions of the battery (this resistance is represented by impedance in electrochemistry), causing a decrease in discharge capacity, i.e., a decrease in capacity. Furthermore, by measuring the impedance of various parts inside the battery (positive electrode, negative electrode, electrolyte), the impact of each part on the battery impedance can be observed (Figure 3). When the temperature is below about -10°C, the interfacial impedance of the positive and negative electrodes (graphite is used as an example in the figure) increases rapidly, while the impedance of the electrolyte begins to rise rapidly around -20°C. The combined result of these impedances manifests as a rapid increase in battery impedance around <-10°C (shown as Li-ion cell in the figure).
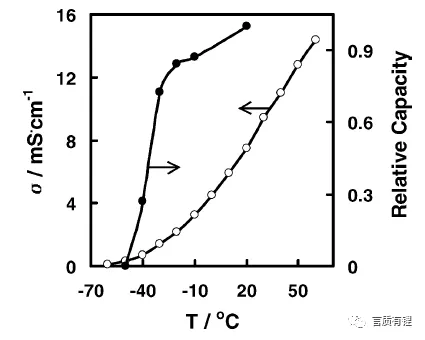
Figure 2: Relationship between Battery Capacity and Electrolyte Conductivity at Different Temperatures
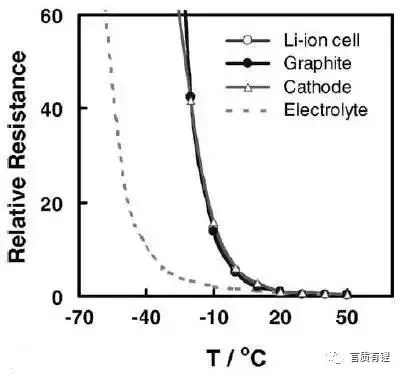
Figure 3: Impedance of Different Parts Inside the Battery at Various Temperatures
Compared to low-temperature discharge, lithium-ion batteries perform even less satisfactorily during low-temperature charging. Charging below 0°C increases the internal pressure of the battery and may cause the safety valve to open. Firstly, charging at low temperatures quickly reaches the constant voltage stage and to some extent reduces the charging capacity while increasing the charging time. Moreover, during low-temperature charging, lithium ions may not have enough time to intercalate into the graphite anode and instead precipitate on the surface of the anode to form lithium metal dendrites. This reaction consumes the lithium ions that can be repeatedly charged and discharged, significantly reducing the battery capacity. The precipitated lithium metal dendrites can also puncture the separator, thereby affecting the safety performance.
The capacity of lithium-ion batteries decreases during low-temperature discharge, but it can be restored after normal temperature charge and discharge, which is a reversible capacity loss. However, low-temperature charging causes lithium plating, which results in permanent capacity loss. Due to the greater harm of lithium plating during low-temperature charging, the management of lithium-ion battery low-temperature charging is stricter than that of low-temperature discharge.
During winter charging, when the outdoor temperature is low and the environment is below 0°C, it is normal for the battery charging speed to decrease or even fail to charge. Please charge the battery in an appropriate ambient temperature to ensure charging effectiveness.
Impact of High Temperatures on Battery Safety Performance
As lithium-ion batteries are increasingly applied to people’s production and daily life, the temperature environment has become a focal point of concern. Relatively speaking, lithium-ion batteries are more prone to safety issues in high-temperature environments. Therefore, it is necessary to test the high-temperature performance of lithium-ion batteries and compare it with their performance at room temperature. When lithium-ion batteries are abused or misused, such as using or charging at high temperatures or charger control failure, it may trigger intense chemical reactions inside the battery, producing a large amount of heat. If the heat does not dissipate in time and rapidly accumulates inside the battery, the battery may leak, release gas, smoke, and in severe cases, it may burn violently and explode.
The chemical reactions that occur in batteries at high temperatures mainly include:
(1) Decomposition of the SEI film: The protective film is metastable and will decompose exothermically at 90-120°C.
(2) Reaction between the embedded lithium and the electrolyte: Above 120°C, the film cannot block the contact between the negative electrode and the electrolyte, and the exothermic reaction occurs between the embedded lithium in the negative electrode and the electrolyte.
(3) Decomposition of the electrolyte: Decomposition and exothermic reaction occur above 200°C.
(4) Decomposition of the positive electrode active material: In the oxidized state, the positive electrode material will exothermically decompose and release oxygen, which reacts exothermically with the electrolyte, or the positive electrode material reacts directly with the electrolyte.
(5) Exothermic reaction between the embedded lithium and the fluoropolymer binder.
The French battery company Saft once studied the impact of high temperatures on battery performance using 2Ah cylindrical batteries (positive electrode material NCM, using PVdF binder, negative electrode material carbon, using CMC/SBR binder) and compared the performance of two batteries at different high temperatures:
B2 battery – first cycled at 60°C for 2 cycles, then cycled at 85°C.
B3 battery – first cycled at 60°C for 2 cycles, then cycled at 120°C.
From Figure 4, it can be seen that after 26 cycles at 85°C, the B2 battery lost about 7.5% of its capacity, and the battery impedance increased by 100%; after 25 cycles at 120°C, the B3 battery lost about 22% of its capacity, and the battery impedance increased by as much as 1115%.
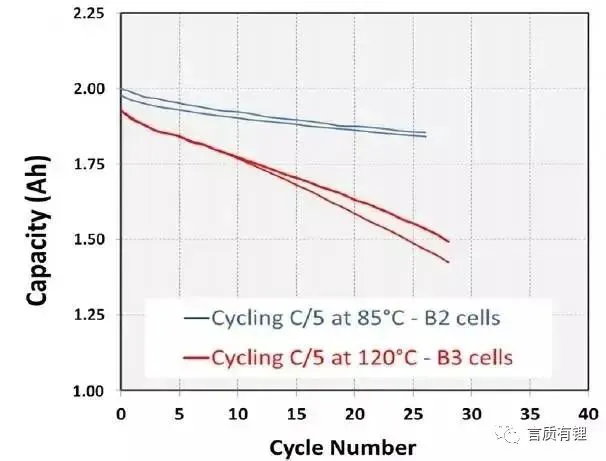
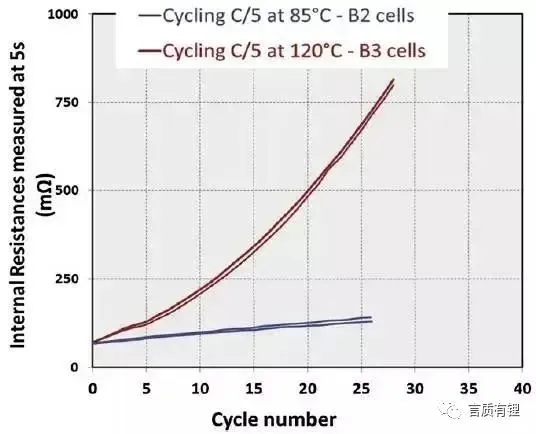
Figure 4: Cycle curves and battery impedance increase curves of B2 and B3 batteries at high temperatures
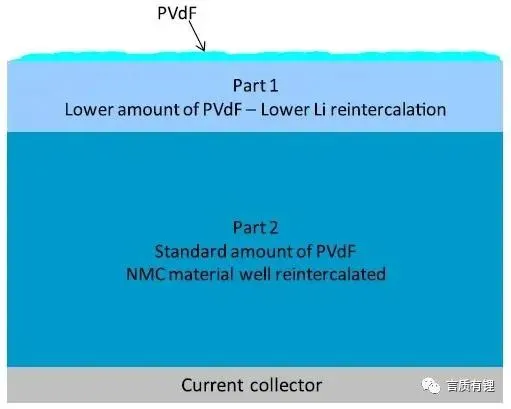
Use the model in Figure 5 to illustrate the changes in the battery cathode at a high temperature of 120°C. At 120°C, some of the cathode binder PVdF migrates from Part 1 to the cathode surface, resulting in a decrease in binder content in Part 1. Due to the lack of binder, the electrochemical reaction capability of the active material NMC decreases. In Part 2, which is the main body of the cathode, the binder content is normal, and the high temperature has little effect, allowing the active material to react normally.
By analyzing the negative electrode surface, the impact of high temperatures on the negative electrode can be observed (Figure 6). Figure 6a shows the initial state of the negative electrode. After cycling at 85°C, the negative electrode surface exhibits common solid electrolyte phases (Figure 6b, where the negative electrode surface is covered with newly generated substances, resulting in a different surface morphology from the initial one, with some small spherical materials. SEI: Solid Electrolyte Interface). When the temperature rises to 120°C, more SEI is generated (Figure 6c, where the negative electrode surface is covered with more particles), consuming more active lithium ions and causing a decrease in capacity.

Figure 6 Morphological changes on the surface of the negative electrode
The Impact of High Temperatures on Battery Life
High operating temperatures: On one hand, they cause the long-term reduction of the electrolyte by the anode at low potentials, resulting in the loss of active lithium ions and a decline in electrochemical performance; on the other hand, high temperatures increase side reactions at the anode, leading to the deposition of inorganic reaction products on the anode surface that hinder the insertion and extraction of lithium ions, accelerating battery aging. At high temperatures, these side reactions increase, such as the decomposition, rupture, or dissolution of the SEI film on the negative electrode surface, causing continuous consumption of lithium ions during cycling and rapid capacity decline.
Ahmad A. Pesaran’s research indicates that when the battery operating temperature exceeds 40°C, the battery’s cycle life halves for every 10°C increase. In the battery packs of new energy vehicles, the tight arrangement of individual cells causes heat accumulation, resulting in temperature differences within the pack, leading to different rates of degradation among individual cells, disrupting the uniformity of the pack, and reducing overall performance.
The temperature of the battery is positively correlated with the charge and discharge current. When charging and discharging with a small current, the highest temperature point of the battery pack is in the middle, where heat exchange with the outside is less likely to occur. When charging and discharging with a large current or when the ear structure design is unreasonable, the highest temperature is at the ear.
Therefore, designing a battery cooling system reasonably according to the characteristics and operating environment of power batteries can not only improve the range performance of the vehicle but also enhance the overall safety and reliability.
The Impact of Temperature Differences on Battery Performance
Battery temperature differences can be categorized into two types: internal temperature differences within a battery, which manifest as temperature uniformity; and temperature differences between individual battery cells, which manifest as temperature consistency.
Causes of internal temperature differences: Generally, during low-temperature heating conditions or high-temperature cooling conditions in water-cooling systems, when a battery module is heated or cooled on one side only, a significant internal temperature difference may occur due to the high thermal resistance of the individual battery cells. This temperature difference is related to the internal structure and material composition of the battery and is difficult to avoid from the perspective of thermal management system design.
Causes of temperature differences between cells: The temperature difference between individual battery cells is mainly determined by the arrangement of the battery module and the structure of the battery thermal management system. This temperature difference can be reduced by optimizing the thermal management design.
Impact of internal temperature differences on individual cells
Excessive internal temperature differences in a battery can cause uneven internal impedance, uneven current distribution, and uneven heat generation, which in turn affect the battery’s performance and accelerate the rate of battery capacity degradation. However, the differences between individual cells are usually small, and thus have a minor impact on consistency.
Impact of temperature differences between cells on the battery
Excessive temperature differences between battery cells can lead to inconsistent performance and capacity degradation rates among the cells within a battery assembly. Since the cells within a battery pack are connected in series, any decline in performance or capacity degradation of a single cell will affect the overall performance of the assembly. Therefore, controlling the consistency of battery temperature is crucial.
Additionally, temperature differences between cells can have a continuous cumulative effect. Cells with higher temperatures age faster, generate more heat, and are more prone to high temperatures.
Conclusion
The operating temperature range of lithium batteries varies depending on the type of lithium battery. Both excessively high and low temperatures can affect the performance of lithium batteries, and in severe cases, may even shorten the battery’s lifespan. To effectively charge, the environmental temperature range for lithium batteries should be between 20-30°C.
In summary, the factors affecting the battery’s performance at high and low temperatures can be summarized as: conductivity of the electrolyte, interfacial impedance, SEI film, etc. These factors collectively affect the battery’s performance. Generally speaking, improving the conductivity or electrical conductivity of the battery components (including choosing more conductive active materials, optimizing electrolyte composition, improving the anode SEI film composition, and suppressing the dissolution of surface materials from the cathode) can help reduce the overall impedance of the battery, which is beneficial for enhancing performance at high and low temperatures. Lithium-ion batteries’ adaptability to temperature is similar to that of the human body; both excessively high and low temperatures are detrimental to their optimal function. Choosing the right materials, optimizing structural design, and customizing appropriate usage conditions are necessary to fully realize their performance.
Source: ATC New Energy Three-Electrics WeChat Public Account
https://mp.weixin.qq.com/s/QNMwYXcf_CiZw2zkjSM16A